Several recent studies have done an excellent job characterizing the architecture of the yeast nuclear pore complex (NPC). With so much new information, researchers are now able to ask probing questions about how NPCs mediate communication between the nucleus and the rest of the cell. Considering that signals perceived from the environment need to reach the transcriptional machinery in the nucleus, and that mRNA transcripts made in response to these signals need to get back out to get translated, the NPC has a lot of communicating to do. A study in a recent issue of the EMBO Journal by Gomar-Alba et al. makes strong strides toward understanding how this communication is accomplished.
On the nuclear side of the NPC resides a substructure called the nuclear basket that has previously been shown to play roles in regulating gene expression and mRNA export. The nuclear basket also interacts with lysine acetyltransferases (KATs) and deacetylases (KDACs) that are best known for modulating transcription via reversible acetylation of histones in chromatin. These enzymes, however, can also act on non-histone proteins and have been linked to numerous cell processes, including DNA damage repair, cell division, and signal transduction.
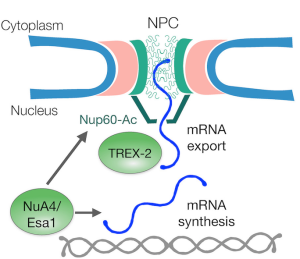
Promotion of mRNA export is another function linked to acetylation, specifically by the NuA4 histone acetyltransferase complex, for which the catalytic subunit is Esa1p. Gomar-Alba et al. show in this recent study that Esa1p is the primary lysine acetyltransferase that promotes cell cycle entry—and also that it acetylates the nuclear pore protein Nup60p.
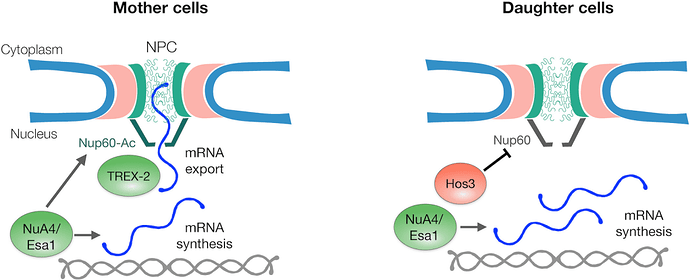
Acetylation of Nup60p promotes mRNA export, which in turn triggers fast entry into the Start phase of the cell cycle, thereby promoting cell division. Nup60p accomplishes this increased export by recruiting the TREX-2 transcription-export complex to the nuclear basket once Nup60p becomes acetylated. The deacetylated form of Nup60p has lower affinity for TREX-2 and thus mRNA export decreases. Deacetylation of Nup60p is performed by Hos3p, which acts in opposition to Esa1p in removing Esa1p-transferred acetyl residues.
Perhaps the most intriguing finding in this study is that Hos3p localizes primarily to daughter cells after cell division, causing displacement of the mRNA export complex and thus slowing G1/S phase transition. This action prevents premature division in the smaller daughter cells, as they require additional growth to meet the size control threshold for entry into a new cell cycle. Accomplishing this level of control with a single enzyme acting on a single nuclear pore protein is a simple, elegant solution.
As usual, studies in yeast make enormous impact on understanding cell division in other organisms.