Regulating mitochondrial dynamics during cell division is crucial for maintaining homeostasis in eukaryotic cells. In budding yeast, proteins such as Myo2p and Mmr1p are essential to transport mitochondria into the growing bud, which post cell division, exists as an independent daughter cell. Improper inheritance and/or distribution of mitochondria can generate reactive oxygen species (ROS) toxicity in daughter cells.
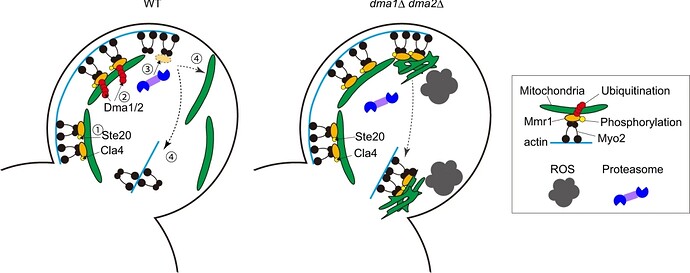
An interesting study by Obara K et al. in Nature Communications shows that degradation of Mmr1p is crucial in maintaining mitochondrial homeostasis in budding yeast. During cell division, Mmr1p bridges mitochondria and Myo1p and assists in its transportation to the bud via actin cables. Once the transportation is complete, kinases such as Ste20p and Cla4p phosphorylate Mmr1, leading to its recognition and poly-ubiquitination by E3 ligases, Dma1p/Dma2p. The proteasome degrades the poly-ubiquitinated and phosphorylated Mmr1p, and the mitochondria are released from Myo2p and distributed in the bud. Once released, Myo2p translocates to the bud neck.
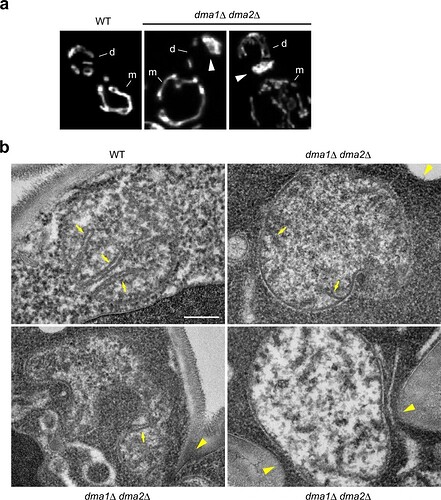
The study shows that in the absence of Dma1p/Dma2p, the transported mitochondria are not released from the actin-myosin machinery but in fact, become expanded or deformed and accumulate at the bud tip and then at the bud neck (due to translocation of Myo2p). Double mutants with altered mitochondrial morphology exhibit elevated respiratory activity and increased generation of ROS, rendering cells hypersensitive to oxidative stress
Additionally, the authors show that bud-localized kinases, Ste20p, and Cla4p phosphorylate (most probably) the serine residue at amino acid 414 of Mmr1p and regulate its degradation only after the mitochondria are transported to the growing bud, a crucial step in proper mitochondrial distribution.
Thus, the study highlights the importance of Mmr1p, Dma1p, and Dma2p in maintaining mitochondrial morphology and distribution required to sustain cell homeostasis in yeast cells.