Eukaryotic transcription is most often initiated by RNA polymerase II (RNAPII) and regulated by several factors, including epigenetic factors, histone modification, DNA methylation, and non-coding antisense transcripts. Non-coding antisense transcripts, produced from the strand opposite to the sense strand, use transcription interference (TI) to regulate gene expression. TI is a phenomenon where one transcription activity negatively impacts the other in cis. One such example is when transcription of long non-coding RNAs (lncRNAs) overlaps with coding gene promoters, causing repression of that gene. Antisense transcripts are involved in several biological processes and show dysregulation in different diseases; however, the mechanisms underlying the antisense mediated transcription interference (AMTI) are not well understood.
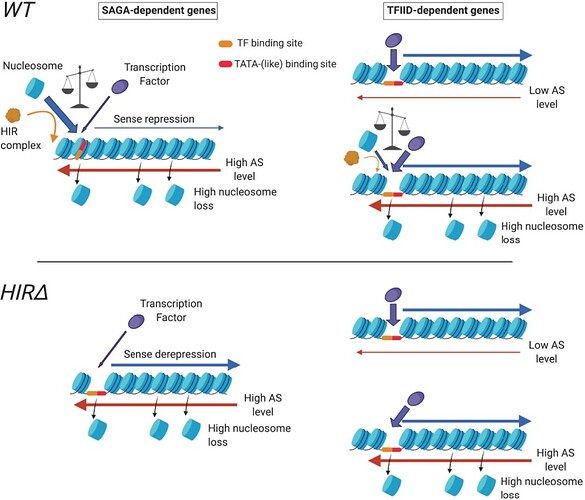
An exciting study by Soudet J et al. in Nucleic Acid Res has identified new components in budding yeast to be involved in AMTI. The authors highlight a strong connection between HIR histone chaperone complex binding and antisense transcription in context with SAGA or TFIID-dependent gene regulation. Likewise, the study shows that induction of antisense transcription influences HIR binding, nucleosome repositioning, and (de novo) histone deposition.
Furthermore, data show that antisense transcription into promoters at SAGA-dependent genes restricts the access of the transcription factor binding sites (TBSs) and transcription factors (TFs) by closing the promoter nucleosome depleted regions (NDRs) with nucleosomes, thus repressing the genes. When antisense elongation begins, nucleosomes are randomly lost from the promoters, and chromatin has a chance to reopen again before new nucleosomes are incorporated. In the absence of HIR complex, the promoters stay open and upregulate the SAGA-dependent genes.
The study confirms that SAGA-dependent genes are associated with higher HIR binding and antisense transcription into promoters. Interestingly, TFIID-dependent genes do not show the same effect even in the presence of high antisense transcription levels. Although SAGA and TFIID-dependent gene classes share a high level of antisense transcription as a common feature, the authors propose that genes can interchange these classes when the balance between TF binding and nucleosome incorporation is changed.
Thus, the study sheds light on the mechanism of the antisense mediated transcription interference and emphasizes the role of the HIR histone chaperone complex in gene regulation.