Messenger RNA (mRNA) 3′ end processing is an evolutionarily conserved and highly controlled process which requires several components from translation/transcription machinery. This processing involves monitoring nascent mRNAs for specific sequences, endonucleolytic cleavage, adding poly(A) tails, and triggering transcription termination. In budding yeast, the 3′ end processing machinery involves the cleavage and polyadenylation factor (CPF) complex and RNA-binding cleavage factors CF IA and CF IB. Based on the different enzymatic roles, the CPF complex has three distinct modules: polymerase, phosphatase, and nuclease.
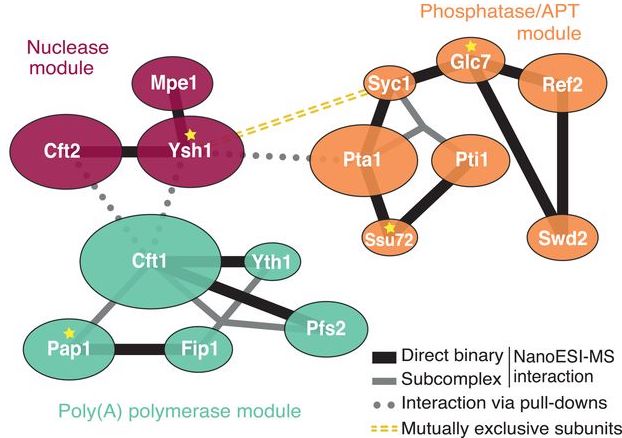
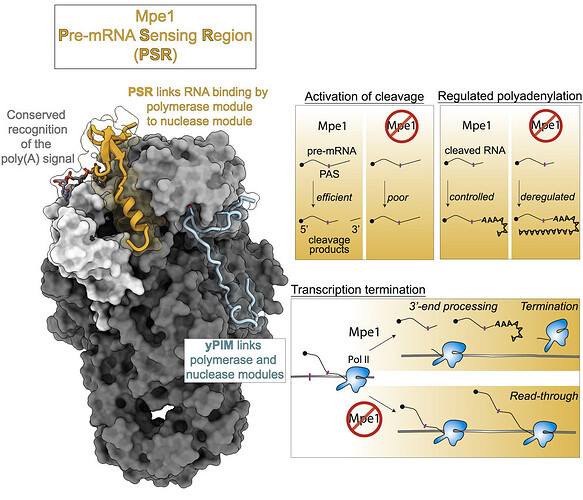
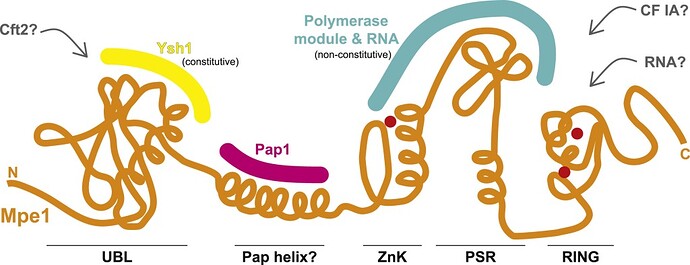
A new study by Rodriguez-Molina et al. in Molecular Cell provides exciting insights into the role of the CPF complex in the 3′ end processing. The study provides strong evidence that Mpe1p, a nuclease module subunit of CPF, is involved in polyadenylation, cleavage, and transcription termination. The data show that in the presence of RNA, Mpe1p directly interacts with the polymerase module subunit Pfs2p through residues 207-268. The authors designate this region as a pre-mRNA-sensing region (PSR) of Mpe1p. However, another nuclease module subunit, Cft2p, hinders this Mpe1-polymerase module interaction.
It is known that the polymerase module subunit Yth1p, interacts with RNA via a polyadenylation signal (PAS), a conserved sequence of A1A2U3A4A5A6. The authors show that in addition to Yth1p, Mpe1 interacts with PAS through the P215 residue. The authors hypothesize that Mpe1-PSR interacts with the polymerase module only after the Yth1p recognizes the PAS RNA, suggesting that Mpe1p may be able to ‘sense’ the RNA-polymerase module binding.
Further, to understand the importance of the PSR region in Mpe1p, the authors analyzed mutants where its interaction with Pfs2p (mpe1-W257A,Y260A) or PAS (mpe1-P215G) is disrupted. Both mutants show reduced endonuclease and polyadenylation activities. Thus, suggesting that the same residues in Mpe1p involved in PSR-RNA/Pfs2 binding are also responsible in activating cleavage and regulating polyadenylation. A similar effect on endonuclease and polyadenylation activities is observed in a mutant where the CPF complex lacks the Mpe1p subunit, further corroborating the role of Mpe1p in mRNA 3′ end processing.
In the CPF complex, Mpe1p interacts with Ysh1p, another nuclease module subunit, via its N-terminal ubiquitin-like domain (UBL). This interaction stabilizes Mpe1p with the nuclease module (and CPF complex) even in the absence of RNA. To evaluate the importance of this interaction, a variant where the UBL region of Mpe1p is disrupted (mpe1-F9A,D45K,R76E,P78G) was generated. This variant is unable to form a stable CPF complex and show deficiencies in activating cleavage and polyadenylation activities. Thus the authors conclude that Mpe1p-Ysh1p interaction is essential for proper processing of 3′ end mRNA.
Another important aspect of mRNA 3′ end processing is the timely termination of transcription. The data show that the CPF complex lacking Mpe1p is unable to successfully terminate the transcription in time. Furthermore, the authors show that the PSR region specifically influences the role of Mpe1p in timely transcription termination.
Thus, the study highlights the role of Mpe1p as an essential subunit of the CPF complex in mRNA 3′ end processing, specifically in cleavage, polyadenylation, and transcription termination.