Several lethal genetic disorders in humans are caused by mutations that cause symptoms of copper (Cu) deficiency, even in the presence of copper. These copper-deficiency disorders are fatal and include Menkes disease, Friedreich’s ataxia, and neurological and cardiac defects in infants due to lack of copper supply to cytochrome c oxidase in mitochondria. Given that no treatments are currently available for these terrible disorders, researchers have been interested in drugs that might improve copper bioavailability. The copper-binding oncological drug elesclomol (ES) has been identified as a candidate.
In a recent report by Garza et al. 2022 in the Journal of Biological Chemistry, the authors use the facility of yeast genetics to ask detailed questions about how ES affects metal homeostasis in yeast cells. It was previously established that perturbation in the levels of one metal tends to cause perturbations in supply of other metals, and thus they asked directed questions about both copper and iron (Fe). Deficiency in copper can cause linked deficiencies in bioavailable iron, and both are critically important for metal-dependent enzymes in mitochondria.
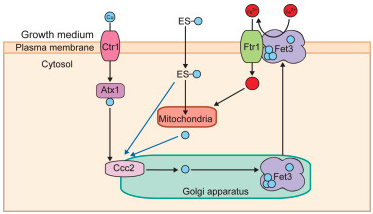
The crux of the team’s findings was that supplementing copper-deficient yeast cells with Cu-bound ES (ES-Cu) not only increased Cu levels, but nearly doubled Fe levels in mitochondria. They were able to show that ES transports copper by an alternate route that bypasses the major yeast copper importer (Ctr1p). While this is an intriguing and perhaps encouraging result, perturbations in metals have so much potential to be toxic that it remains critically important to understand the relationships between the components.
The authors found that application of preformed ES-Cu complex is more efficient than ES at transporting Cu, and that transport of Cu across the plasma membrane by the drug occurs by passive transfusion, not active transport. Further, they made the critical discovery that copper delivered by ES goes first to the Golgi lumen, not directly to mitochondria as the authors had expected based on studies in other models. At the Golgi, the Cu is made available to the copper-transporting ATPase Ccc2p, which in turn assists in metalating Fet3p, a multicopper oxidase that oxidizes ferrous (Fe2+) to ferric iron (Fe3+). Once activated with copper in the Golgi, Fet3p-Cu is transported to the plasma membrane, where it oxidizes iron to Fe3+, the form that can be taken up by the iron transporter Ftr1p. This increased transport of iron leads to increased bioavailability in mitochondria. Thus, the link between copper and iron by means of an alternative copper transporter becomes more clear.
Interestingly, copper and iron metabolism are linked in both humans and yeast. The yeast protein Fet3p has two homologs in humans (ceruloplasmin and hephaestin), both of which are multicopper oxidase proteins critical for normal iron metabolism. From these powerful studies in yeast, it appears that disorders of Cu metabolism cause defects in Fe metabolism due to disrupted metalation of these cuproenzymes within the Golgi. Indeed, this is an intriguing finding that opens avenues for possible therapies, and it would be hard to imagine making this connection without the use of the yeast model.