One way to imagine DNA is as a busy road with a lot of competing traffic. Say, a small village in southern Italy…where someone must mediate conflicts between competing vehicles to avoid disaster.
Illustration by Umberto Aiello, courtesy of the authors
It turns out that the “someone” in yeast cells is Sen1p. Two recent papers from associated groups describe the intriguing detail of how the Sen1p helicase plays this role for RNA polymerase III transcription. The paper by Aiello et al. in Molecular Cell shows how Sen1p regulates transcription-driven conflicts between the several machineries all engaged with DNA. In the related paper by Xie et al. in Science Advances, the authors show how the Sen1p helicase mediates “fail-safe” methods of transcription termination for RNA Pol III, thereby promoting efficiency and avoiding conflict with other pieces of machinery.
From Aiello et al., 2022
The key conflict preventing RNA Pol III from transcribing noncoding genes is with RNA Pol II, which is busy transcribing coding genes. Aiello et al. show how Sen1p has two strategies for mediating these conflicts, both of which involve interactions between Sen1p and the replisome. One involves temporary release of RNA Pol II from DNA while the other resolves genotoxic R-loops in nascent RNA. Both are critical for preventing genome instability.
from Xie et al., 2022
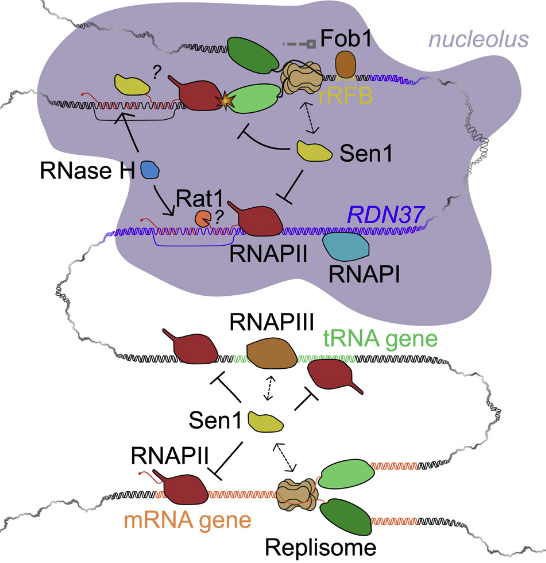
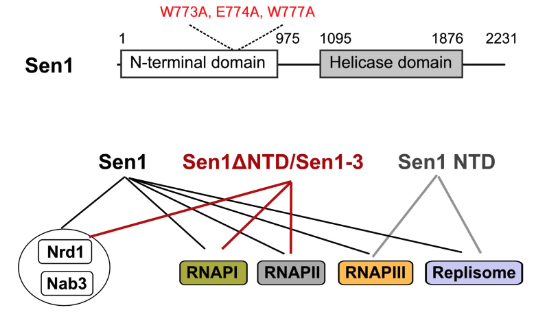
In the related paper by Xie et al., the authors focus on how termination of transcription of noncoding genes by RNA Pol III is achieved, and the role that Sen1p plays in termination. They show how Sen1p can interact with all three polymerases and also with the other two subunits (Nrd1p and Nab3p) of the NRD1 snoRNA termination (NNS) complex. More specifically, they show by mutation and co-immunoprecipitation that it is the N-terminal domain (NTD) of Sen1p that interacts with RNA Pol III and the replisome.
The authors use metagene analysis of RNA Pol II distribution at mRNA-coding genes to show how Sen1p can promote the release of RNA Pol II to resolve transcription-replication conflicts (TRCs). They further show how the association of Sen1p with the replisome is required for limiting TRCs at the ribosomal replication fork barrier, and how this action appears redundant with that of RNases H. The cooperation and redundancy in this role are key means to protect genome stability.
Not only is Sen1p required for termination of RNA Pol III transcription, but the authors show how this function is independent of the NNS complex. Unlike resolution of conflicts between RNA Pol II and RNA Pol III, the termination function of Sen1p does not require the replisome.
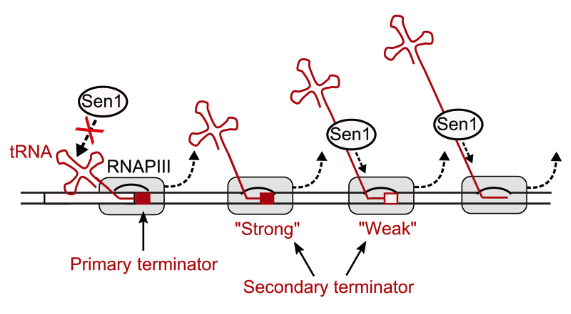
They asked the question of whether Sen1p acts via the primary termination site for RNA Pol III or, rather, a backup secondary termination that catches errors (i.e., when RNA Pol III reads through a weak termination site). Termination for RNA Pol III employs a tract of T nucleotides (T-tract) in the nontemplate strand and these T-tracts can be relatively weak or strong. When T-tracts prove insufficient to stop the polymerase, Sen1p plays a role by means of secondary structures in nascent RNAs, which act as auxiliary cis-acting elements. This backup method is termed the “fail-safe transcription termination pathway.” The RNA secondary structures are not absolutely required for RNAPIII termination, but can function as auxiliary elements that bypass weak or defective termination signals.
from Xie et al., 2022
Once more, it is the power of the yeast model that has allowed investigation to such exquisite molecular detail. That cells preserve genomic stability and avoid pile-ups amid so much traffic along DNA remains truly remarkable–even when we know more of how it works.
