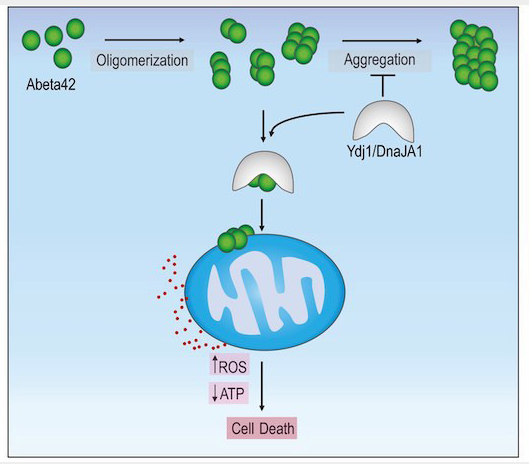
In a 2022 issue of EMBO Molecular Medicine, an intriguing study with potential implications for Alzheimer’s disease (AD) by Ring et al. used yeast to look at why human amyloid beta 42 (Abeta42) kills cells. Upon overexpression in yeast, human Abeta42 protein oligomerizes into aggregates that translocate to mitochondria, where the aggregates cause oxidative stress and eventual necrotic-like cell death. Functional mitochondria are required for this Abeta42-mediated death, and a combined genetic and proteomic approach in yeast identified the HSP40-type chaperone Ydj1p as critical for stabilizing the oligomers and escorting them to mitochondria.
The effect of ydj1Δ deletion was to lower toxicity, with the effect specific to Abeta42 and not to a different type of induced cell death in a yeast model for Parkinson’s disease. Further, Ydj1p protein directly interacted with Abeta42 in a co-immunoprecipitation experiment.
Yeast YDJ1 is homologous to human DnaJA1, which, when expressed in yeast, re-established the toxicity of Abeta42 to a ydj1Δ strain, indicating functional complementation. The human protein directly interacted with Abeta42 in a murine model for Alzheimer’s disease and also displayed dysregulation in post mortem brain samples of AD patients.
In a fly model for AD, deletion of Droj2 (the Drosophila melanogaster ortholog of YDJ1 and DnaJA1) not only reduced the toxicity of Abeta42 but significantly improved the short-term olfactory memory loss associated with Abeta42 expression. Together, the authors convincingly demonstrate the strong evolutionary conservation of this particular chaperone and its effects on exacerbating Abeta42-mediated toxicity, cell death, and memory loss in relevant model systems.
The use of yeast to identify this key factor indicates the power of a simplified and tractable system and will hopefully lead to progress in treating a terrible disease.